Adenosine Triphosphate (ATP) is often referred to as the cell’s main energy currency. It provides energy for living cells so they can carry out all their important life processes. This could include: DNA replication, muscle contraction, cell division, protein synthesis - many different examples!
But ATP doesn’t instantly provide energy for cells - it needs to be broken down through hydrolysis in order to release energy for cells.
Tip
Be careful NOT to say energy is produced. The Law of Conservation of Energy (if you remember back to GCSE Physics), is that energy cannot be created or destroyed, only transferred or transformed. Chemical energy is stored in the bonds of ATP, and is released through hydrolysis. This chemical energy can be transformed into various energy types to support life processes e.g kinetic, heat, mechanical
ATP Structure
Spec Point
Spec Point
A single molecule of adenosine triphosphate (ATP) is a nucleotide derivative and is formed from a molecule of ribose, a molecule of adenine and three phosphate groups.
ATP is made of three components:
- Adenine - Nitrogenous base
- Ribose - 5 carbon ribose sugar
- Phosphate - 3 phosphates (‘tri’ as the name suggests)
It actually has the same structure as an RNA nucleotide (same sugar, base, phosphate — see Structure of DNA & RNA).
All the 3 phosphate groups are negatively charged, which causes them to repel each other. This makes ATP unstable.
The two bonds that link the 3 phosphate groups are high energy bonds (see image below). This means when they are broken in a hydrolysis reaction they release a significant amount of energy.
Hydrolysis & Synthesis of ATP
Spec Point
Spec Point
Hydrolysis of ATP to adenosine diphosphate (ADP) and an inorganic phosphate group (Pi ) is catalysed by the enzyme ATP hydrolase.
- The hydrolysis of ATP can be coupled to energy-requiring reactions within cells.
- The inorganic phosphate released during the hydrolysis of ATP can be used to phosphorylate other compounds, often making them more reactive.
You can think of ATP as a power pack (or a battery is a common analogy for it).
When it’s fully charged you can use it to ‘release energy’ to power other devices (your phone, iPods, etc.) - this is ATP hydrolysis.
But, when it’s run out of charge, it needs to be plugged back in and recharged again - this is ATP synthesis.
Hydrolysis
- The hydrolysis of ATP is catalysed by the enzyme ATP hydrolase (ATPase)
- As this is a hydrolysis reaction it requires water
- A high energy phosphate bond is broken and energy is released, producing ADP and an inorganic phosphate (You don’t need to worry about ‘Inorganic’ phosphate, it just refers to a phosphate that is free and not attached to a carbon. It’s important for cell signalling)
ATP hydrolysis
- ATP hydrolysis can be ‘coupled’ to other energy-requiring reactions within the cell (called energy coupling).
- This ensures that the energy released from ATP hydrolysis can be used by other reactions that need it, and ensures it’s not lost as heat energy.
- A few examples of these energy-coupling reactions include (there are many of these!)
- Nerve cells: Sodium-potassium pump uses ATP to actively transport sodium out of the cells and potassium into the cells, helping to maintain the cell’s resting membrane potential.
- Glycolysis: ATP phosphorylation of glucose to produce glucose phosphate
- Muscle Contraction: ATP is used to move the myosin heads (power stroke) and detach them from the actin-myosin bridge
- What happens to the released phosphate from ATP hydrolysis?
- This phosphate can be added to another compound, known as phosphorylation.
- This can make the other compound more reactive (adding a negatively charged phosphate to the compound).
- An example of this is the phosphorylation of glucose to glucose phosphate, which is a more reactive intermediate in Glycolysis
Synthesis
Spec Point
Spec Point
ATP is resynthesised by the condensation of ADP and Pi . This reaction is catalysed by the enzyme ATP synthase during photosynthesis, or during respiration.
ATP regeneration
- The ATP reaction is reversible so ATP can be resynthesised by the condensation reaction of ADP and Pi, and requires energy (water is released).
- This is catalysed by ATP synthase and happens in:
- Photosynthesis:
- Respiration:
- Oxidative Phosphorylation & Substrate Level Phosphorylation
Exam Question Practice
Describe the structure of ATP.
Outline how named enzymes break down and resynthesise ATP.
(4 marks)Hint
What three components make up ATP? What happens to water in the reactions that make and break ATP?
Answer
Mark Scheme
- Ribose, Adenine and 3 phosphates (1 mark)
- ATP to ADP + Pi by ATP hydrolase in hydrolysis (reaction) (1 mark)
- ADP + Pi to ATP by ATP synthase (1 mark)
- (In) condensation (reaction) (1 mark)
Tips from examiner reports
- Must say ‘ribose’ not ‘pentose’.
- Confusion: Hydrolysis USES water, condensation RELEASES water.
- Tips: ATP = adenine + ribose + 3 phosphates. Know which reaction uses/releases water.
Figure 1 shows a cell from the lining of the ileum specialised for absorption of products of digestion.
SGLT1 is a carrier protein found in the cell-surface membrane of this cell, it transports glucose and sodium ions (Na+) into the cell.
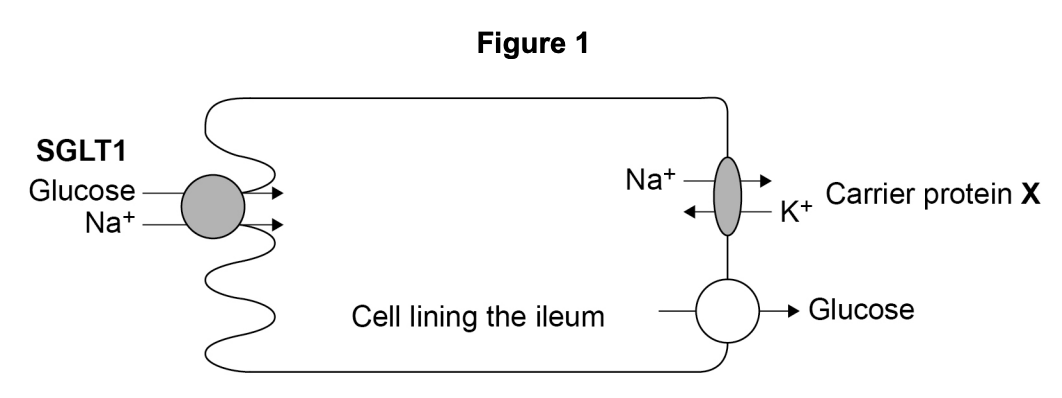
The action of the carrier protein X in Figure 1 is linked to a membrane-bound ATP hydrolase enzyme.
Explain the function of this ATP hydrolase.
(2 marks)Hint
Think about what the carrier protein requires to carry out its role.
Answer
Mark Scheme
- (ATP to ADP + Pi) Releases energy (1 mark)
- (Energy) allows ions to be moved against a concentration gradient OR (energy) allows active transport of ions (1 mark)
Comments from mark scheme
- Reject ‘produces/makes/creates energy’
- For ‘ions’ accept Na+ or K+
- Do not accept if this movement is of glucose not ions
Scientists investigated the action of the enzyme ATP synthase. They made reaction mixtures each containing:
- ATP synthase
- buffer (to control pH)
- substrates
One of the substrates required in these reaction mixtures is inorganic phosphate (Pi).
Select one box to show which other substrate the scientists must add to the reaction mixtures to produce ATP.
Scientists investigated treatment of a human bladder infection caused by a species of bacterium. This species of bacterium is often resistant to the antibiotics currently used for treatment.
They investigated the use of a new antibiotic to treat the bladder infection. The new antibiotic inhibits the bacterial ATP synthase enzyme.
Select the appropriate box next to the equation which represents the reaction catalysed by ATP synthase.
Comments from mark scheme